All information is correct at time of printing and is subject to change without notice. The Devon Formulary and Referral Website is not in any way liable for the accuracy of any information printed and stored by users. For the most up-to-date information, please refer to the website.

Formulary
Page last updated:
12 February 2025
Contact us about this page
6.4.1 Female sex hormones and their modulators
Click here for a visual summary of systemic HRT treatment options. |
Menopause Advice & Guidance Service, click here: South Devon / West Devon |
Vaginal oestrogen preparations to be used in the relief of vaginal atrophy can be found here 7.2.1 Preparations for vaginal and vulval changes
Further guidance supporting the prescribing of HRT preparations and the clinical management of menopause can be found here: Guidance on the management of menopause.
For women without a uterus or cervix, oestrogen-only HRT is appropriate, however, in endometriosis, endometrial foci may remain despite hysterectomy and the addition of a progestogen should be considered in these circumstances.
For women with a uterus, oestrogen plus progestogen HRT is required. In women with a subtotal hysterectomy (retaining their cervix), oestrogen plus progesterone may be required*. A progestogen should be added to reduce the risk of cystic hyperplasia of the endometrium and possible transformation to cancer.
- For use in the perimenopause or within 12 months of the last menstrual period, a sequential (cyclical) combined regimen is recommended.
- For postmenopausal women whose last menstrual period occurred over 12 months previously, a continuous combined regimen is recommended.
* There is limited evidence to guide practice in relation to the role or need for progestogen replacement in women who have had subtotal hysterectomy. British Menopause Society guidance suggests considering oestrogen plus sequential progestogen for up to 3 months:
- If this results in monthly bleeding, endometrial cells are present and are responding to the hormones, therefore oestrogen plus progestogen HRT should be used thereafter. If a woman would like to avoid monthly bleeding, continuous combined HRT is advised.
- If there is no bleeding, it is unlikely that residual endometrium is present and oestrogen only HRT can be used.
The following table outlining the progestogen dose that appears to provide adequate endometrial protection for different strengths of licensed oestrogen doses is reproduced from the British Menopause Society (BMS) guideline on management of unscheduled bleeding on HRT (April 2024).
- Please note norethisterone is not currently recommended for this indication locally.
- A number of the doses / regimens in this table are off-label (refer to individual product entries for licensed indications & doses).
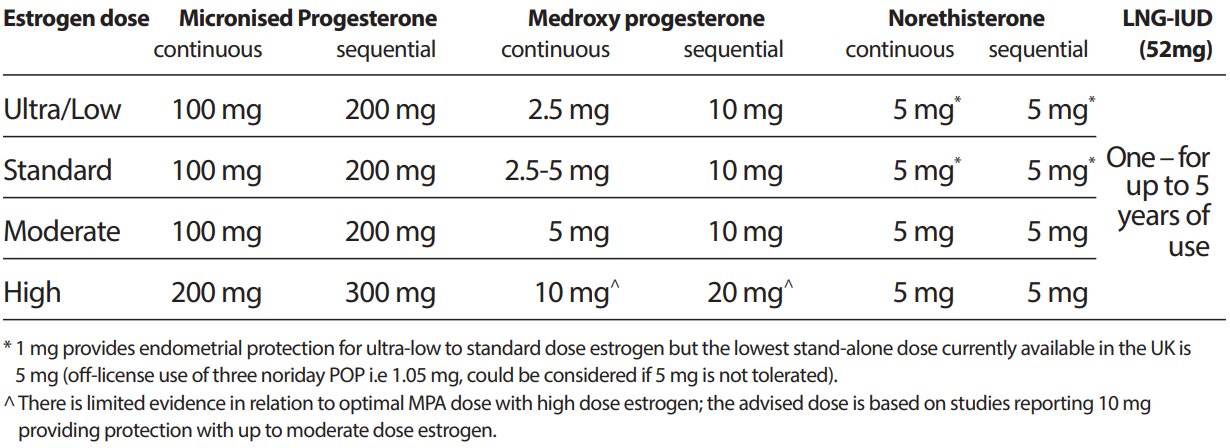
The following table classifying oestrogen doses is reproduced from the British Menopause Society (BMS) guideline on management of unscheduled bleeding on HRT (April 2024). Refer to individual product entries for licensed indications and doses.
- Please note Sandrena gel is non-formulary
- A number of the doses in this table are off-label (refer to individual product entries for licensed indications & doses).
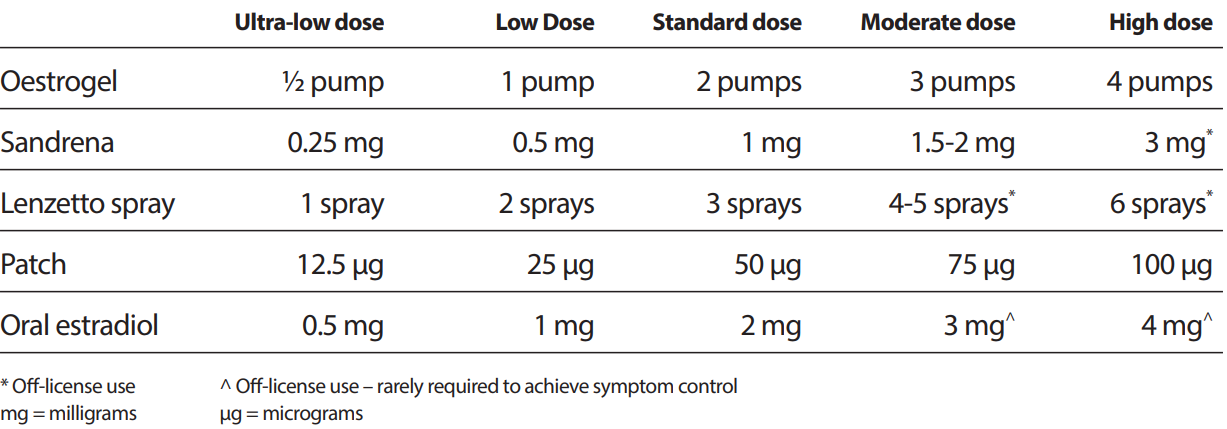
Route of administration
Consider transdermal rather than oral HRT for menopausal women who are at increased risk of venous thromboembolism (VTE) including those with a BMI over 30kg/m2. Consider referring those at high risk (strong family history of VTE or a hereditary thrombophilia) to a haematologist for assessment before considering HRT.
The transdermal route should also be considered when oral treatment is not sufficiently effective or is not tolerated, or if the individual has cardiovascular risk factors (e.g. obesity, uncontrolled hypertension, or hypertriglyceridaemia) or a gastrointestinal disorder that may affect absorption of oral treatment, or has a history of migraine or gallbladder disease, or is taking concomitant hepatic enzyme-inducing drug treatment.
Visual summary
Click here for a visual summary of systemic HRT treatment options.
Oestrogen only
Women with a uterus require addition of a progestogen (see above).
Oral
Elleste solo
(Estradiol)
- Tablets 1mg, 2mg (£5.06 = 84 tablets)
Indications and dose
- Menopausal symptoms
- 1mg daily, increased if necessary to 2mg daily
- Osteoporosis prophylaxis in postmenopausal women
- 2mg daily
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients).
- Start treatment on day 1 of menstruation (or at any time if cycles have ceased or are infrequent).
- For women with a uterus, oestrogen plus progestogen HRT is required. In women with a subtotal hysterectomy (retaining their cervix), oestrogen plus progesterone may be required (see introductory section above).
Transdermal
Consider transdermal rather than oral HRT for menopausal women who are at increased risk of VTE, including those with a BMI over 30kg/m2. Consider referring those at high risk (strong family history of VTE or a hereditary thrombophilia) to a haematologist for assessment before considering HRT.
The transdermal route should also be considered when oral treatment is not sufficiently effective or is not tolerated, or if the individual has cardiovascular risk factors (e.g. obesity, uncontrolled hypertension, or hypertriglyceridaemia) or a gastrointestinal disorder that may affect absorption of oral treatment, or has a history of migraine or gallbladder disease, or is taking concomitant hepatic enzyme-inducing drug treatment.
Transdermal patches
Evorel (1st line transdermal)
(Estradiol)
- Transdermal patches 25micrograms/24hours (£4.07 = 8 patches), 50micrograms/24hours (£4.62 = 8 patches), 75micrograms/24hours (£4.90 = 8 patches), 100micrograms/24hours (£5.09 = 8 patches)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women (minimum 50micrograms/24hours)
Dose
- Apply 1 patch twice weekly continuously
- Start within 5 days of onset of menstruation (or at any time if cycles have ceased or are infrequent)
- For women with a uterus, oestrogen plus progestogen HRT is required. In women with a subtotal hysterectomy (retaining their cervix), oestrogen plus progesterone may be required (see introductory section above)
- Therapy should be initiated with Evorel 50 patch; subsequently adjust according to response; dose may be reduced to Evorel 25 patch after first month if necessary for menopausal symptoms only
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients).
- Evorel is first line in patients for whom the transdermal route is clinically indicated (see above).
Estradot (2nd line transdermal)
(Estradiol)
- Transdermal patches 25micrograms/24hours (£7.38 = 8 patches), 37.5micrograms/24hours (£7.39 = 8 patches), 50micrograms/24hours (£7.41 = 8 patches), 75micrograms/24hours (£8.62 = 8 patches), 100micrograms/24hours (£8.95 = 8 patches)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women (minimum 50micrograms/24hours)
Dose
- Apply 1 patch twice weekly continuously.
- Start within 5 days of onset of menstruation (or at any time if cycles have ceased or are infrequent).
- For women with a uterus, oestrogen plus progestogen HRT is required. In women with a subtotal hysterectomy (retaining their cervix), oestrogen plus progesterone may be required (see introductory section above).
- For menopausal symptoms, therapy should be initiated with Estradot 25 patch for first 3 months; subsequently adjust according to response.
- For osteoporosis prophylaxis, therapy should be initiated with Estradot 50 patch; subsequently adjust according to response.
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients)
Transdermal gel
Recommended as alternative option where the transdermal route is clinically indicated but Evorel transdermal patches are ineffective, not tolerated, or impractical.
Oestrogel Pump-Pack (2nd line transdermal)
(Estradiol)
- Transdermal gel 0.06%, providing 750micrograms estradiol per 1.25g pump actuation (£6.17 = 80g (64 metered doses))
Indications and dose
- Menopausal symptoms
- Apply 1.5mg (2 pumps) once daily continuously, increased if necessary up to 3mg (4 pumps) once daily after 1 month of continuous treatment.
- Osteoporosis prophylaxis in postmenopausal women
- Apply 1.5mg (2 pumps) once daily continuously.
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients)
- If switching from cyclical HRT, start at end of regimen; otherwise start at any time.
- For women with a uterus, oestrogen plus progestogen HRT is required. In women with a subtotal hysterectomy (retaining their cervix), oestrogen plus progesterone may be required (see introductory section above).
- To be applied over a large area. Apply gel to clean, dry, intact skin such as arms, shoulders or inner thighs and allow to dry for 5 minutes before covering with clothing. Not to be applied on or near breasts or on vulval region. Avoid skin contact with another person (particularly male) and avoid other skin products or washing the area for at least 1 hour after application.
Transdermal spray
Recommended as alternative option where the transdermal route is clinically indicated but transdermal patches (Evorel or Estradot) and Oestrogel are ineffective, not tolerated, or impractical.
Lenzetto (3rd line transdermal)
(Estradiol)
- Transdermal spray, 1.53mg/spray (£6.90 = 56 sprays)
Indications and dose
- Menopausal symptoms
- Apply 1 spray once daily continuously, increased if necessary to 2 sprays once daily after at least 4 weeks of continuous treatment.
- Maximum dose 3 sprays daily
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients).
- For women with a uterus, oestrogen plus progestogen HRT is required. In women with a subtotal hysterectomy (retaining their cervix), oestrogen plus progesterone may be required (see introductory section above).
- To be applied over a large area. Apply to dry, healthy skin of the inner forearm or alternatively the inner thigh and allow to dry for 2 minutes before covering with clothing. Avoid skin contact with another person (particularly children) or pets and avoid washing the area for at least 1 hour after application. If a sunscreen is needed, apply at least 1 hour before Lenzetto.
- Lenzetto is not licensed or recommended for osteoporosis prophylaxis.
Oestrogen with progestogen
Continuous Combined Preparations
Continuous combined preparations are not suitable for use in the perimenopause or within 12 months of the last menstrual period.
Continuous combined regimens do not produce withdrawal bleeding, however irregular bleeding or spotting may occur in the first 4-6 months of treatment. If bleeding persists beyond 6 months of initiating (or 3 months after changing HRT preparation), becomes heavier, or occurs after a spell of amenorrhoea, endometrial pathology should be excluded, and consideration given to adjusting HRT. Refer to the British Menopause Society (BMS) guideline on management of unscheduled bleeding on HRT (April 2024) and the following Clinical Referral Guidelines:
Oral
Kliofem
(Estradiol with norethisterone)
- Tablets 2mg/1mg (£11.43 = 84 tablets)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women
Dose
- Women with a uterus whose last menstrual period occurred over 12 months previously: 1 tablet daily continuously; if changing from cyclical HRT begin treatment at the end of scheduled bleed.
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients)
Kliovance
(Estradiol with norethisterone)
- Tablets (1mg/500microgram) (£13.20 = 84 tablets)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women
Dose
- Women with a uterus whose last menstrual period occurred over 12 months previously: 1 tablet daily continuously; start at end of scheduled bleed if changing from cyclical HRT
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients)
- Kliovance has a lower norethisterone content
Bijuve
(Estradiol with micronised progesterone)
- Capsules (1mg/100mg) (£24.42 = 84 tablets)
Indications
- Menopausal symptoms
Dose
- Women whose last menstrual period occurred over 12 months previously, 1 tablet daily continuously, to be taken in the evening with food (if changing from cyclical HRT begin treatment the day after finishing the 28-day cycle)
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients)
- Bijuve is recommended for patients experiencing troublesome progestogenic side effects with first line options.
Femoston Conti
(Estradiol with dydrogesterone)
- Tablets (0.5mg/2.5mg), (1mg/5mg) (£24.43 = 84 tablets)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women (1mg/5mg strength only)
Dose
- Women whose last menstrual period occurred over 12 months previously, 1 tablet daily continuously (if changing from cyclical HRT begin treatment the day after finishing oestrogen plus progestogen phase)
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients)
- Femoston products are included for patients experiencing troublesome progestogenic side effects with first line options.
Transdermal
Consider transdermal rather than oral HRT for menopausal women who are at increased risk of VTE, including those with a BMI over 30kg/m2. Consider referring those at high risk (strong family history of VTE or a hereditary thrombophilia) to a haematologist for assessment before considering HRT.
The transdermal route should also be considered when oral treatment is not sufficiently effective or is not tolerated, or if the individual has cardiovascular risk factors (e.g. obesity, uncontrolled hypertension, or hypertriglyceridaemia) or a gastrointestinal disorder that may affect absorption of oral treatment, or has a history of migraine or gallbladder disease, or is taking concomitant hepatic enzyme-inducing drug treatment.
Evorel Conti
(Estradiol with norethisterone)
- Transdermal patches (50micrograms/170micrograms/24hours) (£44.28 = 24 patches)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women
Dose
- 1 patch to be applied twice weekly continuously, to be started at end of scheduled bleed if changing from cyclical HRT.
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients).
- Evorel Conti is first line in patients for whom the transdermal route is clinically indicated (see above)
FemSeven Conti
(Estradiol with levonorgestrel)
- Transdermal patches (50micrograms/7micrograms/24hours) (£44.12 = 12 patches)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women
Dose
- 1 patch to be applied once weekly continuously.
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients).
Sequential Combined Preparations (Cyclical HRT)
Sequential combined preparations are suitable for use in the perimenopause or within 12 months of the last menstrual period. They usually result in withdrawal bleeding at the end of each course of progestogen.
BMS guidance recommends that women taking sequential HRT over the age of 45 should be offered, after five years of use or by age 54 (whichever comes first), a change to continuous combined HRT.
Oral
Elleste Duet
(Estradiol with norethisterone)
- Tablets 1mg and (1mg+1mg) (£9.20 = 84 tablets)
- Tablets 2mg and (2mg+1mg) (£9.20 = 84 tablets)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women (2mg tablets only)
Dose
- 1 tablet (estradiol) daily for 16 days starting on day 1 of menstruation (or at any time if cycles have ceased or are infrequent), then 1 tablet (estradiol and norethisterone) daily for 12 days; subsequent courses are repeated without interval
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients)
Femoston
(Estradiol with dydrogesterone)
- Femoston 1/10 tablets 1mg and (1mg+10mg) (£16.16 = 84 tablets)
- Femoston 2/10 tablets 2mg and (2mg+10mg) (£16.16 = 84 tablets)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women
Dose
- 1 tablet (estradiol) daily for 14 days, starting within 5 days of onset of menstruation (or any time if cycles have ceased or are infrequent) then 1 tablet (estradiol and dydrogesterone) daily for 14 days; subsequent courses repeated without interval
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients)
- Femoston products are recommended for patients experiencing troublesome progestogenic side effects with first line options.
Transdermal
Consider transdermal rather than oral HRT for menopausal women who are at increased risk of VTE, including those with a BMI over 30kg/m2. Consider referring those at high risk (strong family history of VTE or a hereditary thrombophilia) to a haematologist for assessment before considering HRT.
The transdermal route should also be considered when oral treatment is not sufficiently effective or is not tolerated, or if the individual has cardiovascular risk factors (e.g. obesity, uncontrolled hypertension, or hypertriglyceridaemia) or a gastrointestinal disorder that may affect absorption of oral treatment, or has a history of migraine or gallbladder disease, or is taking concomitant hepatic enzyme-inducing drug treatment.
Evorel Sequi
(Estradiol with norethisterone)
- Transdermal patches 50micrograms/24hours and 50micrograms/170micrograms/24hours (£13.20 = 8 patches) (see note 3)
Indications
- Menopausal symptoms
- Osteoporosis prophylaxis in postmenopausal women
Dose
- One Evorel 50 patch to be applied twice weekly for 2 weeks, starting within 5 days of onset of menstruation (or at any time if cycles have ceased or are infrequent), followed by one Evorel Conti patch twice weekly for 2 weeks; subsequent courses are repeated without interval.
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients)
- Evorel Sequi is first line in patients for whom the transdermal route is clinically indicated (see above)
- Each Evorel Sequi box contains 4 x Evorel 50 patches (estradiol only) and 4 x Evorel Conti patches (estradiol with norethisterone).
Progestogens
Progestogens act mainly on tissues sensitised by oestrogens and are used for menstrual disorders including severe dysmenorrhoea and menorrhagia.
Refer to 7.3.2.3 Intrauterine progestogen-only devices for levonorgestrel intrauterine devices (LNG-IUD) for contraception, menorrhagia and prevention of endometrial hyperplasia during oestrogen HRT.
Gepretix
(Micronised progesterone)
- Oral capsules 100mg (£4.62 = 30 capsules)
Indications and dose
- Progestogenic opposition of oestrogen HRT:
- Sequential (cyclical) regimen: 200mg once daily on days 15–26 of each 28-day oestrogen HRT cycle. (licensed dose).
- BMS also recommends an alternative (off-label) sequential regimen of 200mg once daily for two weeks per 28 day cycle may help to reduce administration and prescribing errors.
- BMS recommends higher (off-label) doses may be required for women taking high dose oestrogen (refer to tables at top of page)
- Continuous regimen: 100mg once daily on days 1–25 of each 28-day oestrogen HRT cycle (licensed dose).
- BMS also recommends an alternative (off-label) continuous regimen of 100mg once daily continually.
- BMS recommends higher (off-label) doses may be required for women taking high dose oestrogen (refer to tables at top of page)
- Dose to be taken at bedtime (may cause drowsiness / dizziness)
- Sequential (cyclical) regimen: 200mg once daily on days 15–26 of each 28-day oestrogen HRT cycle. (licensed dose).
Notes
- Prescribe by brand (to aid identification where products contain multiple ingredients, or to prevent confusion where multiple brands contain similar ingredients).
- Concomitant food ingestion increases the bioavailability of micronised progesterone.
- Off-label use of Gepretix intravaginally is not supported.
- Gepretix 100 mg soft capsules contain soybean lecithin and may cause hypersensitivity reactions (urticarial and anaphylactic shock in hypersensitive patients). As there is a possible relationship between allergy to soya and allergy to peanut, patients with peanut allergy should avoid using Gepretix 100mg soft capsules.
- For vaginal micronised progesterone for threatened miscarriage, refer to 7.1.4 Prevention of miscarriage.
Medroxyprogesterone acetate
- Tablets 2.5mg (£1.84 = 30 tablets), 5mg (£3.69 = 30 tablets), 10mg (£7.39 = 30 tablets)
- Tablets 100mg, 200mg, 400mg (only for cancer indications)
Indications and dose
- Dysfunctional uterine bleeding:
- 2.5–10mg daily for 5–10 days, repeated for 2 cycles, begin treatment on day 16 to 21 of cycle
- Secondary amenorrhoea:
- 2.5–10mg daily for 5–10 days, repeated for 3 cycles, begin treatment on day 16 to 21 of cycle
- Mild to moderate endometriosis:
- 10mg 3 times daily for 90 consecutive days, beginning on day 1 of cycle.
- Progestogenic opposition of oestrogen HRT (second line) (off-label)
- Sequential (cyclical) regimen: 10mg daily for the last 14 days of each 28-day oestrogen HRT cycle (BNF)
- BMS recommends higher doses may be required for women taking high dose oestrogen (refer to tables at top of page)
- Continuous regimen: 2.5-10mg once daily continually depending on oestrogen dose (refer to tables at top of page)
- Sequential (cyclical) regimen: 10mg daily for the last 14 days of each 28-day oestrogen HRT cycle (BNF)
- Doses used for the management of cancer are much higher – refer to specialist for guidance.
Notes
- Refer also to 7.3.2.2 Parenteral progestogen-only contraceptives (for Depo-Provera intramuscular injection for contraception)
- Healthcare Professional Communication (October 2024): Medroxyprogesterone acetate: Risk of meningioma and measures to minimise this risk
- There is a small increased risk of developing meningioma with high doses of medroxyprogesterone acetate (all injectable formulations and ≥100mg oral formulations), primarily after prolonged use (several years).
- For oncological indications: If a meningioma is diagnosed, the need to continue the treatment should be carefully reconsidered, on a case-by-case basis taking into account individual benefits and risks.
Norethisterone
- Tablets 5mg (£6.58 = 30 tablets)
Indications and dose
- Endometriosis:
- 10–15mg daily for 4–6 months or longer, starting on day 5 of cycle (if spotting occurs increase dose to 20–25mg daily, reduced once bleeding has stopped)
- Dysfunctional uterine bleeding or menorrhagia:
- 5mg 3 times daily for 10 days to arrest bleeding; 5mg twice daily from day 19 to 26 of cycle to prevent bleeding
- Dysmenorrhoea:
- 5mg 3 times daily from day 5 to 24 of cycle, for 3–4 cycles
- Postponement of menstruation:
- 5mg 3 times daily starting 3 days before expected onset (menstruation occurs 2–3 days after stopping)
Notes
- The norethisterone 5mg preparation is not licensed or recommended for HRT.
- Refer also to 7.3.2 Progestogen-only contraceptives (for oral norethisterone for contraception).
Dienogest
- Tablets 2mg (£20.50 = 2mg daily)
Indications and dose
- Endometriosis (see note 1)
- One tablet daily
- Dienogest 2mg/day is not a contraceptive (see note 3)
Notes:
- Dienogest is accepted for use in the management of endometriosis when initial hormonal therapy (for example, the combined oral contraceptive pill or a progestogen) is ineffective or not tolerated or contraindicated.
- Treatment initiation:
- A trial period (e.g. 4 to 6 months) is required to establish effectiveness and tolerability.
- Treatment with dienogest may be started in primary care on the advice of a specialist.
- Contraception: Any hormonal contraception needs to be stopped prior to initiation of dienogest. Dienogest 2mg/day has not been tested for contraceptive efficacy. If contraception is required, non-hormonal methods of contraception should be used (e.g. barrier method) (SmPC).
- Treatment can be started on any day of the menstrual cycle. Tablets must be taken continuously without regard to vaginal bleeding and preferably at the same time each day.
- Missed tablets / gastrointestinal disturbance: refer to SmPC.
- In patients who are at an increased risk of osteoporosis a careful risk-benefit assessment should be performed before starting dienogest. Endogenous estrogen levels are moderately decreased during treatment with dienogest.
- The majority of patients treated with 2mg dienogest experience changes in their menstrual bleeding pattern. Undesirable effects are more common during the first months after the start of treatment with dienogest, and subside with continued treatment.
Progesterone
- Vaginal gel 90mg (8%) / application (£30.83 = 15 x 1.125g applicators)
- Pessaries 200mg, 400mg (£8.95, £12.96 = 15 pessaries)
- Injection 50mg/1ml, 100mg/2ml
Notes
- For threatened miscarriage, refer to 7.1.4 Prevention of miscarriage.
Hydroxyprogesterone caproate
- Injection 250mg/ml (unlicensed preparation)
Noretynodrel derivatives
Tibolone is not suitable for use in the perimenopause or within 12 months of the last menstrual period; women who use such preparations may bleed irregularly in the early stages of treatment—if bleeding continues endometrial abnormality should be ruled out, and consideration given to adjusting HRT. Refer to the British Menopause Society (BMS) guideline on management of unscheduled bleeding on HRT (April 2024) and the following Clinical Referral Guidelines:
For women older than about 60 years, the risks associated with tibolone start to outweigh the benefits because of the increased risk of stroke (see note 2 below).
Tibolone
Tablets 2.5mg (£2.89)
Indications
- Short-term treatment of symptoms of oestrogen deficiency (including women being treated with gonadotrophin releasing hormone analogues)
- Osteoporosis prophylaxis in women at risk of fractures (second-line when other prophylaxis contra-indicated or not tolerated)
Dose
- 2.5mg daily
Notes
- Tibolone combines oestrogenic and progestogenic activity with weak androgenic activity; it is given continuously, without cyclical progestogen.
- The benefit-risk balance for tibolone, is described separately to conventional combined HRT. Refer to the September 2007 MHRA drug safety update, the BNF and the manufacturer's summary of product characteristics (SmPC) for details.
Selective oestrogen receptor modulators
NICE CG164: Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer (July 2013, updated Nov 2019)
- Unless the patient has a past history or may be at increased risk of thromboembolic disease:
- Consider raloxifene for 5 years for postmenopausal women with a uterus at moderate or high risk of breast cancer who have severe osteoporosis or do not wish to take anastrozole or tamoxifen
Raloxifene
- Tablets 60mg (£5.26 = 60mg once daily)
Indications and dose
- Secondary prevention of osteoporotic fragility fractures in postmenopausal women
- Adult: 60mg daily
- Chemoprevention of moderate-to-high risk breast cancer in women (off-label) (refer to NICE CG164)
- Adult: 60mg daily for 5 years
Notes
- Raloxifene does not reduce menopausal vasomotor symptoms
- Women taking raloxifene are at increased risk of thromboembolism
- NICE TA160: Raloxifene is not recommended as a treatment option for the primary prevention of osteoporotic fragility fractures in postmenopausal women (February 2018)
- NICE TA161: Raloxifene is recommended as an alternative treatment option for the secondary prevention of osteoporotic fragility fractures in postmenopausal women (February 2018)
- who are unable to comply with the special instructions for the administration of alendronate and risedronate, or have a contraindication to or are intolerant of alendronate and risedronate and
- who also have a combination of T-score, age and number of independent clinical risk factors for fracture as indicated in NICE TA161
Progesterone receptor modulators
Ulipristal acetate
- Tablets 5mg
Indications
- Up to 4 courses of intermittent treatment of moderate to severe symptoms of uterine fibroids in adult women who have not reached when uterine fibroid embolisation and/or surgical treatment options are not suitable or have failed (see note 4)
Notes
- Please refer to section 7.3.5 Emergency Contraception for use of ulipristal acetate in emergency contraception
- Periodic monitoring of the endometrium including annual ultrasound is recommended
- The routine commissioning of ulipristal acetate 5mg tablets (Esmya) is accepted in Devon for up to 4 courses of intermittent treatment of moderate to severe symptoms of uterine fibroids in adult women who have not reached menopause when uterine fibroid embolisation and/or surgical treatment options are not suitable or have failed (see Commissioning Policy for more details)
- MHRA Drug Safety Update (February 2021): Ulipristal acetate 5mg (Esmya): further restrictions due to risk of serious liver injury (see below):
Advice for healthcare professionals
- Ulipristal acetate 5mg for uterine fibroids has been associated with cases of serious liver injury and liver failure (requiring transplantation in some cases); the licence was temporarily suspended in March 2020 to allow a further review of these risks
- Although the temporary suspension has been lifted, the indication for ulipristal acetate 5mg has been further restricted – it should be used only for intermittent therapy of moderate to severe uterine fibroid symptoms before menopause and when surgical procedures (including uterine fibroid embolisation) are not suitable or failed
- Ulipristal acetate 5mg should no longer be prescribed for controlling symptoms of uterine fibroids while waiting for surgical treatment
- If ulipristal acetate 5mg is felt to be an appropriate therapy, talk about the risks and benefits with patients before prescribing so they can make an informed decision about treatment options; this conversation should include discussion of:
- all available treatment options for moderate to severe symptoms of uterine fibroids, and the advantages and risks of these depending on personal situation
- the potential risk of liver injury and liver failure with ulipristal acetate 5mg, which in rare cases has led to liver transplantation
- signs and symptoms of liver injury and what to do if they occur
- Continue to follow advice to monitor liver function according to the recommended schedule of liver function tests before, during, and after treatment courses. The prescriber is responsible for ensuring drug safety monitoring is undertaken
Reminder of liver function monitoring
- Ulipristal acetate 5mg is contraindicated in patients with an underlying hepatic disorder.
- Liver function tests must be performed before starting treatment with ulipristal acetate 5mg. Treatment must not be initiated if transaminases (alanine transaminase (ALT) or aspartate aminotransferase (AST)) exceed 2-times the upper limit of normal (ULN).
- During treatment, liver function tests must be performed monthly during the first 2 treatment courses. For further treatment courses, liver function must be tested once before each new treatment course and when clinically indicated.
- If a patient shows signs or symptoms compatible with liver injury (fatigue, asthenia, nausea, vomiting, right hypochondrial pain, anorexia, jaundice), treatment should be stopped, and the patient investigated immediately. Liver function tests should be performed urgently.
- Stop treatment if transaminase levels (ALT or AST) are greater than 3-times the ULN and closely monitor patients. The need for specialist hepatology referral should be considered.
- Liver function tests should also be performed 2–4 weeks after treatment has stopped
Advice to give to patients:
- Infrequent but serious cases of liver damage (with some cases requiring a liver transplant) have been reported in association with ulipristal acetate 5mg for uterine fibroids
- Ulipristal acetate 5mg can only be used for intermittent treatment of moderate to severe symptoms of uterine fibroids if:
- you have not experienced menopause; and
- an operation or embolisation procedure for uterine fibroids is not suitable for you or these procedures have not worked
- Blood tests are needed before treatment is started, during treatment, and 2–4 weeks after treatment to check your liver is functioning normally
- Stop taking your ulipristal acetate tablets and speak with your doctor immediately if you get any signs of liver damage such as yellowing of the skin or eyes, dark urine, or nausea or vomiting
