All information is correct at time of printing and is subject to change without notice. The Devon Formulary and Referral Website is not in any way liable for the accuracy of any information printed and stored by users. For the most up-to-date information, please refer to the website.

Formulary
Page last updated:
9 December 2024
Contact us about this page
Management of Chronic Obstructive Pulmonary Disease (COPD)
Click here for a visual summary of inhaled therapy for COPD. |
The following recommendations are largely based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for Prevention, Diagnosis and Management of Chronic Obstructive Pulmonary Disease (COPD) 2024 Report. Where recommendations have been made by NICE (2019) this is indicated in the text. The information is supported by local respiratory specialists and is intended to guide and rationalise treatment choices when managing patients with COPD. Diagnosis is not covered in the guidance below. It is important to establish that patients meet the diagnostic criteria for COPD before commencing treatment.
Non inhaled prevention and maintenance strategies are essential in the management of COPD (see slider below)
Therapy should be reviewed annually and following an exacerbation. Treatment regimens should be patient-specific and individualised. Patient preference should be considered when prescribing treatments. It is essential that patients can demonstrate the proper inhaler technique when prescribing an inhaler device; recheck patient technique at each visit to ensure continued correct use of the inhaler. Adherence to treatment regimens should also be checked. When discussing inhaled treatment options, consideration should also be given to the environmental impact of inhalers. Local and national resources which support patient training can be accessed via links at the bottom of the page.
Key:
- DPI = dry powder inhaler; SMI = soft mist inhaler; pMDI = pressurised metered dose inhaler; BAI = breath actuated inhaler
- SABA = short-acting beta2 agonist; SAMA = short-acting muscarinic antagonist; LABA = long-acting beta2 agonist; LAMA = long-acting muscarinic antagonist; ICS = inhaled corticosteroid
- MRC = Medical Research Council breathlessness / dyspnoea scale; CAT = COPD Assessment Test
A guide to GOLD 2024
Non-inhaled prevention and maintenance strategies including smoking cessation, vaccinations and pulmonary rehabilitation play a vital role in the management of COPD – see slider below for further details.
Initial inhaled therapy: Initial pharmacological management of COPD should be carried out according to the individualised assessment of symptoms and exacerbation risk and subsequent categorisation to one of three groups A, B, or E:
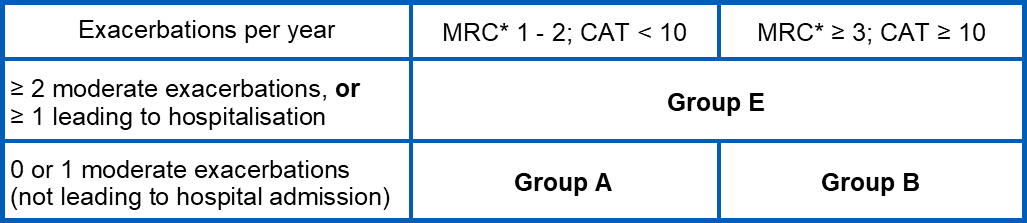
* Note: GOLD 2024 refers to the modified MRC (mMRC) scale, which produces scores from 0 to 4; however, Devon formulary guidance utilises the standard MRC scale, which uses almost identical questions but has a range of scores from 1 to 5, and is routinely collected by GPs as part of the COPD indicator for the national Quality and Outcomes Framework (QOF).
For more information on the Medical Research Council (MRC) breathlessness / dyspnoea scale, click here
For more information on the COPD Assessment Test (CAT), click here
Follow-up inhaled therapy: Following implementation of therapy, patients should be reassessed for attainment of treatment goals and identification of any barriers for successful treatment. Consideration should be given to inhaler technique and adherence and non-inhaled approaches. Follow-up recommendations are intended to facilitate management of patients taking maintenance treatment(s) either early after initial treatment or after years of follow-up.
Following review, adjustments in treatment may be required.
Follow-up treatment does not depend on the GOLD group (A, B or E) allocated at treatment initiation.
Consider the predominant treatable trait to target: dyspnoea or exacerbations. Use exacerbation pathway if both exacerbations and dyspnoea need to be targeted.
See follow-up maintenance inhaled therapy sliders below.
ICS + LABA combination therapy
The use of ICS + LABA combination inhalers is no longer encouraged in patients with COPD. If a patient with COPD and no features of asthma is well controlled on ICS + LABA, continuation with this treatment is an option. However, if the patient has:
- Controlled symptoms and has never had raised eosinophils ≥ 0.3x109/L: consider switching to LABA + LAMA combination therapy at next review
- Persistent breathlessness or exercise limitation: consider switching treatment to LABA + LAMA combination therapy.
- Further exacerbations:
- switch to LABA + LAMA combination therapy, unless triple therapy is indicated (see below)
- step up treatment to ICS + LABA + LAMA triple combination therapy if:
- blood eosinophils ≥ 0.3x109/L, or
- ≥ 1 severe exacerbation (requiring hospitalisation) or
- ≥ 2 moderate exacerbations in the last year
Visual Summary
Click here for a visual summary of inhaled therapy for COPD.
Revisit the following treatments and plans at every review.
Smoking cessation
Smoking cessation has the greatest capacity to influence the natural history of COPD, it also improves daily symptoms and decreases the frequency of exacerbations.
At every opportunity, advise and encourage every person with COPD who is still smoking (regardless of their age) to stop, and offer them help to do so.
To optimise the results from smoking cessation programmes it is advised that patients access motivational support from specialist services.
Refer all people who smoke to a smoking cessation service for behavioural support and pharmacotherapy. Some local community pharmacies and GP practices may offer a level 2 service. Level 3 services can be accessed via:
See 4.10.2 Nicotine dependence for formulary product listings and further information.
Vaccinations
Pneumococcal vaccination and annual influenza vaccination should be offered to all patients with COPD.
Formulary guidance on the use of vaccines and the treatment of influenza is available.
Self-management plans
Self-management education and the issuing of a written action plan have been shown to improve outcomes for COPD.
Self-management plans should be individualised and written in collaboration with the patient with COPD and the family and carers (as appropriate). Self-management plans should be reviewed regularly.
Patients at risk of an exacerbation of COPD should be given an exacerbation action plan that encourages them to respond promptly to the symptoms of an exacerbation (see Management of acute exacerbations below).
Local COPD self-management plans are available here:
Pulmonary rehabilitation
Pulmonary rehabilitation includes education, exercise and self-management intervention, intended to improve symptoms, quality of life and physical and emotional participation in everyday activities. It is individually tailored and designed to optimise each person's physical and social performance and autonomy.
GOLD recommends pulmonary rehabilitation for all patients in groups B and E (see above). NICE recommends pulmonary rehabilitation for patients who consider themselves functionally disabled by COPD (usually Medical Research Council [MRC] grade 3 and above), including those who have had a recent hospitalisation for an acute exacerbation.
Pulmonary rehabilitation is not suitable for patients who are unable to walk, have unstable angina or who have had a recent myocardial infarction.
Click here for a visual summary of inhaled therapy for COPD.
Rescue short-acting bronchodilators should be prescribed to all patients for immediate symptom relief.
See section 3.1.1 Adrenoceptor agonists and 3.1.2 Antimuscarinic bronchodilators
Group A typical presentation
- 0 or 1 moderate exacerbation per year, without hospitalisation
- CAT score <10
- MRC grade 1-2
Recommended inhaled treatment
Bronchodilator therapy: GOLD (2024) indicates that long-acting bronchodilators (LAMAs or LABAs) are preferred over short-acting agents, except for patients with only occasional dyspnoea and for immediate relief of symptoms in patients already on long-acting bronchodilators.
Local specialists recommend LAMA therapy as the preferred option. GOLD (2024) indicates that clinical trials have shown a greater effect on exacerbation rates for LAMA (tiotropium) versus LABA treatment.
LAMA monotherapy:
Tiogiva (tiotropium, DPI)
- Contents of one capsule inhaled once daily, or
Spiriva Respimat (tiotropium, SMI)
- Two inhalations once daily, or
Seebri Breezhaler (glycopyrronium bromide, DPI)
- Contents of one capsule inhaled once daily, or
Eklira Genuair (aclidinium bromide) (DPI)
- One inhalation once daily
See section 3.1.2 Antimuscarinic bronchodilators
If LAMA monotherapy is not suitable, consider LABA monotherapy (section 3.1.1 Adrenoceptor agonists)
Review
Following implementation of therapy, patients should be re-assessed for attainment of treatment goals and any barriers to successful treatment.
Treatment reviews should:
- Assess adherence with existing therapies
- Assess inhaler technique, spacer use and possible side effects
- Assess non-inhaled prevention and maintenance strategies (see above)
Continue treatment if benefit documented. If a change in treatment is considered, consider the predominant treatable trait to target: dyspnoea or exacerbations for follow-up maintenance therapy as shown below. Use exacerbation pathway if both exacerbations and dyspnoea need to be targeted.
Follow-up treatment does not depend on the GOLD group allocated at treatment initiation.
Click here for a visual summary of inhaled therapy for COPD.
Rescue short-acting bronchodilators should be prescribed to all patients for immediate symptom relief.
See section 3.1.1 Adrenoceptor agonists and 3.1.2 Antimuscarinic bronchodilators
Group B typical presentation
- 0 or 1 moderate exacerbation per year, without hospitalisation
- CAT score ≥10
- MRC grade ≥ 3
Recommended inhaled treatment
LABA plus LAMA combination inhaler:
Anoro Ellipta (vilanterol / umeclidinium bromide, DPI),
- One inhalation once daily, or
Ultibro Breezhaler (indacaterol / glycopyrronium bromide, DPI)
- Contents of one capsule inhaled once daily, or
Spiolto Respimat (olodaterol / tiotropium bromide, SMI)
- Two inhalations once daily, or
Bevespi Aerosphere (formoterol / glycopyrronium bromide, pMDI)
- Two inhalations twice daily via spacer
See section 3.1.4 Combination inhalers
Review
Following implementation of therapy, patients should be re-assessed for attainment of treatment goals and any barriers to successful treatment.
Treatment reviews should:
- Assess adherence with existing therapies
- Assess inhaler technique, spacer use and possible side effects
- Assess non-inhaled prevention and maintenance strategies (see above)
Continue treatment if benefit documented. If a change in treatment is considered, consider the predominant treatable trait to target: dyspnoea or exacerbations for follow-up maintenance therapy as shown below. Use exacerbation pathway if both exacerbations and dyspnoea need to be targeted.
Follow-up treatment does not depend on the GOLD group allocated at treatment initiation.
Click here for a visual summary of inhaled therapy for COPD.
Rescue short-acting bronchodilators should be prescribed to all patients for immediate symptom relief.
See section 3.1.1 Adrenoceptor agonists and 3.1.2 Antimuscarinic bronchodilators
Group E typical presentation
- At least 2 moderate exacerbations per year, or at least one leading to hospitalisation (regardless of MRC grade / CAT score)
Recommended inhaled treatment
LABA plus LAMA combination inhalers are recommended, however if blood eosinophils ≥ 0.3x109/L, consider ICS plus LABA plus LAMA triple combination inhaler.
LABA plus LAMA combination inhaler:
Anoro Ellipta (vilanterol / umeclidinium bromide, DPI),
- One inhalation once daily, or
Ultibro Breezhaler (indacaterol / glycopyrronium bromide, DPI)
- Contents of one capsule inhaled once daily, or
Spiolto Respimat (olodaterol / tiotropium bromide, SMI)
- Two inhalations once daily, or
Bevespi Aerosphere (formoterol / glycopyrronium bromide, pMDI)
- Two inhalations twice daily via spacer
See sections 3.1.4 Combination inhalers
ICS plus LABA plus LAMA triple combination inhaler:
(Only consider for initial therapy if blood eosinophils ≥ 0.3x109/L)
Trelegy Ellipta (fluticasone furoate / vilanterol / umeclidinium, DPI)
- One inhalation once daily, or
Trimbow NEXThaler (beclomethasone / formoterol / glycopyrronium bromide, DPI)
- Two inhalations twice daily, or
Trimbow pMDI (beclomethasone / formoterol / glycopyrronium bromide, pMDI)
- Two inhalations (87 micrograms / 5 micrograms / 9 micrograms)* twice daily via spacer, or
Trixeo Aerosphere (budesonide / formoterol / glycopyrronium bromide, pMDI)
- Two inhalations twice daily via spacer
See section 3.1.4 Combination inhalers
* Please note, the higher strength Trimbow pMDI (172 micrograms / 5 micrograms / 9 micrograms) is not recommended (or licensed) for COPD; it is also non-formulary.
Review
Following implementation of therapy, patients should be re-assessed for attainment of treatment goals and any barriers to successful treatment.
Treatment reviews should:
- Assess adherence with existing therapies
- Assess inhaler technique, spacer use and possible side effects
- Assess non-inhaled prevention and maintenance strategies (see above)
Continue treatment if benefit documented. If a change in treatment is considered, consider the predominant treatable trait to target: dyspnoea or exacerbations for follow-up maintenance therapy as shown below. Use exacerbation pathway if both exacerbations and dyspnoea need to be targeted.
NICE recommends use of ICS should be reviewed at least annually and the reason for ongoing ICS use documented in the patient's medical records. De-escalation guidance is detailed below.
Follow-up treatment does not depend on the GOLD group allocated at treatment initiation.
De-escalation
Response to treatment escalation should always be reviewed; consider de-escalation if there is a lack of clinical benefit and/or side effects occur. De-escalation may also be considered in patients receiving treatment who return with resolution of some symptoms that subsequently require less therapy.
De-escalation should be undertaken carefully, ensuring that patients are aware of how to report decline in symptoms.
ICS should be discontinued if there has been a lack of response or patient experiencing side effects (including pneumonia); see “Inhaled corticosteroids and pneumonia” below.
Click here for a visual summary of inhaled therapy for COPD.
Follow-up treatment does not depend on the GOLD group allocated at treatment initiation.
Exacerbation pathway should be used if both exacerbations and dyspnoea need to be targeted.
Before initiating a new drug therapy practitioners should:
- Assess adherence with existing therapies
- Assess inhaler technique, spacer use and possible side effects
- Assess non-inhaled prevention and maintenance strategies (see above)
- Consider other causes of breathlessness such as heart failure or atrial fibrillation.
Persistent breathlessness or exercise limitation despite LAMA (or LABA) monotherapy
Step up to LABA plus LAMA combination inhaler.
LABA plus LAMA combination inhaler:
Anoro Ellipta (vilanterol / umeclidinium bromide, DPI),
- One inhalation once daily, or
Ultibro Breezhaler (indacaterol / glycopyrronium bromide, DPI)
- Contents of one capsule inhaled once daily, or
Spiolto Respimat (olodaterol / tiotropium bromide, SMI)
- Two inhalations once daily, or
Bevespi Aerosphere (formoterol / glycopyrronium bromide, pMDI)
- Two inhalations twice daily via spacer
See section 3.1.4 Combination inhalers
Review patient:
- If persistent symptoms see further options below
Persistent breathlessness or exercise limitation despite LABA plus LAMA combination inhaler
Guidelines differ on their approach to managing these patients, and there is no strong evidence to provide clear direction.
GOLD recommends:
- considering a switch of inhaler device or molecules (to a different LABA plus LAMA combination inhaler)
- implementing or escalating non-pharmacological treatment(s)
- investigating and treating other causes of dyspnoa
NICE recommends stepping up from LABA plus LAMA to ICS plus LABA plus LAMA triple combination inhaler if day-to-day symptoms continue to adversely impact patient's quality of life.
Alternative LABA plus LAMA combination inhaler (GOLD, 2024):
Anoro Ellipta (vilanterol / umeclidinium bromide, DPI),
- One inhalation once daily, or
Ultibro Breezhaler (indacaterol / glycopyrronium bromide, DPI)
- Contents of one capsule inhaled once daily, or
Spiolto Respimat (olodaterol / tiotropium bromide, SMI)
- Two inhalations once daily, or
Bevespi Aerosphere (formoterol / glycopyrronium bromide, pMDI)
- Two inhalations twice daily via spacer
See section 3.1.4 Combination inhalers
OR
ICS plus LABA plus LAMA triple combination inhaler (NICE, 2019):
Trelegy Ellipta (fluticasone furoate / vilanterol / umeclidinium, DPI)
- One inhalation once daily, or
Trimbow NEXThaler (beclomethasone / formoterol / glycopyrronium bromide, DPI)
- Two inhalations twice daily, or
Trimbow pMDI (beclomethasone / formoterol / glycopyrronium bromide, pMDI)
- Two inhalations (87 micrograms / 5 micrograms / 9 micrograms)* twice daily via spacer, or
Trixeo Aerosphere (budesonide / formoterol / glycopyrronium bromide, pMDI)
- Two inhalations twice daily via spacer
See section 3.1.4 Combination inhalers
* Please note, the higher strength Trimbow pMDI (172 micrograms / 5 micrograms / 9 micrograms) is not recommended (or licensed) for COPD; it is also non-formulary.
Review patient:
- Review triple therapy after 3 months and if no improvement in symptoms revert back to LABA plus LAMA combination inhaler and explore further treatment options such as those described in Other pharmacological treatments below.
- NICE recommends use of ICS should be reviewed at least annually and the reason for ongoing ICS use documented in the patient's medical records.
- De-escalation guidance is detailed below
De-escalation
Response to treatment escalation should always be reviewed; consider de-escalation if there is a lack of clinical benefit and/or side effects occur. De-escalation may also be considered in patients receiving treatment who return with resolution of some symptoms that subsequently require less therapy.
De-escalation should be undertaken carefully, ensuring that patients are aware of how to report decline in symptoms.
ICS should be discontinued if there has been a lack of response or patient experiencing side effects (including pneumonia); see "Inhaled corticosteroids and pneumonia" below.
Click here for a visual summary of inhaled therapy for COPD.
Follow-up treatment does not depend on the GOLD group allocated at treatment initiation.
Exacerbation pathway should be used if both exacerbations and dyspnoea need to be targeted.
Before initiating a new drug therapy practitioners should:
- Assess adherence with existing therapies
- Assess inhaler technique, spacer use and possible side effects
- Assess non-inhaled prevention and maintenance strategies (see above)
Persistent exacerbations despite LAMA (or LABA) monotherapy
Step up to LABA plus LAMA combination inhaler.
LABA plus LAMA combination inhaler:
Anoro Ellipta (vilanterol / umeclidinium, DPI),
- One inhalation once daily, or
Ultibro Breezhaler (indacaterol / glycopyrronium bromide, DPI)
- Contents of one capsule inhaled once daily, or
Spiolto Respimat (olodaterol / tiotropium bromide, SMI)
- Two inhalations once daily, or
Bevespi Aerosphere (formoterol / glycopyrronium bromide, pMDI)
- Two inhalations twice daily via spacer
See section 3.1.4 Combination inhalers
Persistent exacerbations despite LABA plus LAMA combination inhaler
- Step up to ICS plus LABA plus LAMA triple combination inhaler (a beneficial response after the addition of ICS may be observed at blood eosinophil counts ≥ 0.1x109/L, with a greater magnitude of response more likely with higher eosinophil counts), or
- Refer to Respiratory Consultant for consideration of other treatment options including azithromycin and roflumilast (see Other pharmacological treatments below).
NICE recommends stepping up from LABA plus LAMA combination inhaler to triple therapy if day-to-day symptoms continue to adversely impact patient's quality of life, if they have a severe exacerbation (requiring hospitalisation), or they have 2 moderate exacerbations within a year.
ICS plus LABA plus LAMA triple combination inhaler:
Trelegy Ellipta (fluticasone furoate / vilanterol / umeclidinium, DPI)
- One inhalation once daily, or
Trimbow NEXThaler (beclomethasone / formoterol / glycopyrronium bromide, DPI)
- Two inhalations twice daily, or
Trimbow pMDI (beclomethasone / formoterol / glycopyrronium bromide, pMDI)
- Two inhalations (87 micrograms / 5 micrograms / 9 micrograms)* twice daily via spacer, or
Trixeo Aerosphere (budesonide / formoterol / glycopyrronium bromide, pMDI)
- Two inhalations twice daily via spacer
See section 3.1.4 Combination inhalers
* Please note, the higher strength Trimbow pMDI (172 micrograms / 5 micrograms / 9 micrograms) is not recommended (or licensed) for COPD; it is also non-formulary.
Review patient:
- NICE recommends use of ICS should be reviewed at least annually and the reason for ongoing ICS use documented in the patient's medical records.
- De-escalation guidance is detailed below
Persistent exacerbations despite ICS plus LABA plus LAMA triple combination inhaler
- Refer to Respiratory Consultant for consideration of other treatment options including azithromycin and roflumilast (see "Other pharmacological treatments" below).
- Stop ICS if there are adverse effects (including pneumonia) or a reported lack of efficacy.
De-escalation
Response to treatment escalation should always be reviewed; consider de-escalation if there is a lack of clinical benefit and/or side effects occur. De-escalation may also be considered in patients receiving treatment who return with resolution of some symptoms that subsequently require less therapy.
De-escalation should be undertaken carefully, ensuring that patients are aware of how to report decline in symptoms.
ICS should be discontinued if there has been a lack of response or patient experiencing side effects (including pneumonia); see "Inhaled corticosteroids and pneumonia" below.
Physicians should remain vigilant for pneumonia and other infections of the lower respiratory tract (i.e. bronchitis) in patients with COPD who are treated with inhaled products that contain steroids because the clinical features of such infections and exacerbations frequently overlap (see Lower respiratory tract infections).
Any patient with severe COPD who has had pneumonia during treatment with ICS should have their treatment reconsidered.
NICE recommends use of an ICS should be reviewed at least annually and the reason for ongoing ICS use documented in the patient's medical records.
Oral steroids
The use of oral corticosteroids as maintenance treatment is not generally recommended. In a few patients with advanced COPD maintenance treatment with oral steroids may be needed if they cannot be withdrawn after an exacerbation. In these cases the dose should be kept as low as possible and consideration given to osteoporosis prophylaxis (see 6.6 Drugs affecting bone metabolism).
Oral theophylline
Theophylline exerts a small bronchodilator effect in stable COPD, associated with modest symptomatic benefits. Plasma levels and interactions need to be monitored (see 3.1.3 Theophylline)
Mucolytic therapy
Mucolytic therapy can be considered for patients with a chronic productive cough and continued only if there is symptomatic improvement following a 4-week trial (see 3.7 Mucolytics).
Do not routinely use mucolytic drugs to prevent exacerbations in people with stable COPD (NICE NG115).
Oral prophylactic antibiotic therapy
Seek specialist Advice & Guidance (where available) or refer to respiratory consultant before commencing prophylactic antibiotic therapy.
NICE makes the following recommendations regarding the use of prophylactic oral antibiotics.
Before offering prophylactic antibiotics, ensure that the person has been considered for:
- sputum culture and sensitivity (including tuberculosis culture), to identify other possible causes of persistent or recurrent infection that may need specific treatment (for example, antibiotic-resistant organisms, atypical mycobacteria or Pseudomonas aeruginosa)
- training in airway clearance techniques to optimise sputum clearance
- a CT scan of the thorax to rule out bronchiectasis and other lung pathologies
Consider azithromycin (usually 250mg 3 times a week) for at least three months for people with COPD if they:
- do not smoke and
- have optimised non-pharmacological management and inhaled therapies, relevant vaccinations and (if appropriate) have been referred for pulmonary rehabilitation and
- continue to have 1 or more of the following, particularly if they have significant daily sputum production:
- frequent (typically 4 or more per year) exacerbations with sputum production
- prolonged exacerbations with sputum production
- exacerbations resulting in hospitalisation.
Note: Azithromycin is not licensed for use in this indication.
Before starting azithromycin, ensure the person has had an electrocardiogram (ECG) to rule out prolonged QT interval and baseline liver function tests. Advise people about the small risk of hearing loss and tinnitus associated with azithromycin, and tell them to contact a healthcare professional if this occurs.
Review prophylactic azithromycin after the first 3 months, and then at least every 6 months. Only continue treatment if the continued benefits outweigh the risks. Be aware that there are no long-term studies on the use of prophylactic antibiotics in people with COPD.
For people who are taking prophylactic azithromycin and are still at risk of exacerbations, provide a non-macrolide antibiotic to keep at home as part of their COPD rescue pack (see "Management of acute exacerbations" below).
It is not necessary to stop prophylactic azithromycin during an acute exacerbation of COPD.
See section 5.1.5 Macrolides
Roflumilast
Refer to respiratory consultant for consideration of roflumilast (hospital only) in line with NICE TA461
See section 3.3.3 Phosphodiesterase type-4 inhibitors
Nebulisers
NICE recommends considering nebuliser therapy for people with distressing or disabling breathlessness despite maximum therapy with inhalers, and continue only if there is an improvement in symptoms, daily living activities, exercise capacity or lung function.
For more information see Nebulisation guidance
Oxygen therapy
Be aware that inappropriate use of oxygen therapy in patients with COPD may cause respiratory depression.
Formulary oxygen guidance including the Home Oxygen Order Form (HOOF) can be found here.
Devon Clinical Referral Guidelines (CRGs) on the use of oxygen can be found here:
COPD exacerbations are most commonly caused by respiratory tract infections. Symptoms usually last between 7 – 10 days, but some events may last longer.
The severity of the exacerbation and severity of the underlying disease will determine if it is managed in the inpatient or outpatient setting.
Bronchodilators
- SABAs with or without SAMAs are recommended as the initial bronchodilators to treat increased breathlessness during an acute exacerbation.
- Increase doses and/or frequency. It is recommended that patients use a metered dose inhaler one puff every hour for two or three doses and then every 2 – 4 hours based on response.
- Use spacers or air-driven nebulisers when appropriate. Do not use nebulisers continuously. Change patients back to hand-held inhalers as soon as the condition has stabilised.
- Continue long-acting bronchodilators during exacerbation or initiate as soon as possible before hospital discharge.
Glucocorticoids
- Consider prednisolone 30mg-40mg daily for 5 days in patients with a significant increase in breathlessness that interferes with daily activities and in all patients admitted to hospital unless contra-indicated (see 6.3.2 Glucocorticoid therapy) .
- Consider osteoporosis prophylaxis for patients taking frequent courses of oral corticosteroids (see 6.6 Drugs affecting bone metabolism).
Antibiotics
- GOLD 2024 recommends antibiotics for COPD exacerbations for:
- patients who have three cardinal symptoms: increased dyspnoea, sputum volume and sputum purulence
- patients with two of the above symptoms, if sputum purulence is one of the symptoms
- patients requiring mechanical ventilation (invasive or non-invasive)
- Improvements in dyspnoea and sputum purulence suggest clinical success
- See Lower Respiratory Tract Infections for further guidance and choice of antibiotics in acute COPD exacerbations
COPD Rescue Packs
Patients who have had an exacerbation within the last year and remain at risk of having an exacerbation should be given a COPD rescue pack of antibiotic and corticosteroid tablets to keep at home for use as part of their exacerbation action plan, if they understand and are confident about when and how to take these medicines (see Non inhaled prevention and maintenance strategies, above). Patients must be reminded to request replacement packs when used or expired.
Important: Please note these information leaflets are only relevant to prescriptions for standby supply of antibiotics and corticosteroid prescribed as described here, this is due to the specific nature of information contained regarding drugs and their doses.
Opioids can be used for the palliation of breathlessness in patients with end stage COPD unresponsive to other medical therapy in consultation with a specialist.
Use benzodiazepines, tricyclic antidepressants, major tranquilisers and oxygen where appropriate. Involve multidisciplinary palliative care teams and hospices.
- Visual summary of inhaled therapy for COPD
- NHS Devon respiratory resources for health care professionals
- NHS Devon greener respiratory care resources
- Smoke Free Devon – Local information for GPs
-
Smoking cessation services:
- 2024 GOLD Report
- NICE COPD resources
- RightBreathe.com – a website which contains resources to aid patient education and training on use of inhalers
NICE has produced a patient decision aid to help people with asthma and their healthcare professionals discuss their options for inhaler devices (available here); it is suitable for use by people aged 17 years and over, and many of the considerations are also applicable to patients with COPD.
Advice on how to obtain placebo inhalers can be obtained from the NHS Devon Medicines Optimisation Team, please contact: d-icb.medicinesoptimisation@nhs.net
